Recent changes in management
Key recent changes in the management of HFrEF are increased usage of sacubitril + valsartan (Entresto) and the introduction of the sodium- glucose co-transporter-2 inhibitors, dapagliflozin or empagliflozin, into treatment schedules.
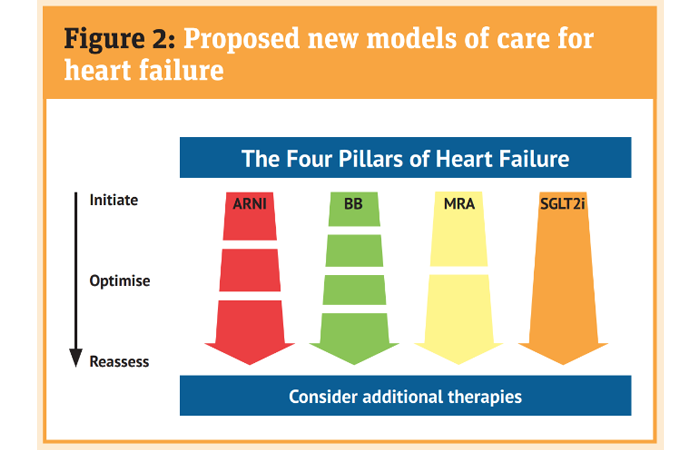
The traditional stepwise approach described earlier is increasingly being questioned with specialists preferring to ‘personalise’ the introduction of medicines and sequencing to establish patients on ‘four pillar therapy’ for HFrEF (Figure 2).
Treatment options
Sacubitril + valsartan (Entresto) was approved by NICE following the publication of the PARADIGM trial, which showed a reduction in mortality and an improvement in quality of life compared with enalapril (an ACEI).
Sacubitril is a neprilysin inhibitor (NI). It acts to reduce the damage to the heart resulting from increased natriuretic peptides that are released when the heart muscle is over-stretched. It is prescribed in combination with the ARB valsartan and the combination is known as an ARNI.
Sacubitril + valsartan is an alternative to treatment with an ACEI or any other ARB. When starting the treatment any ACEI must be stopped for at least 36 hours before the first dose of sacubitril + valsartan is given as there is a risk of angioedema if they are co-prescribed. Any other ARB needs to be discontinued as the product contains valsartan but a wash-out period is not required.
Careful monitoring of blood pressure (more potent effect than ACEI/ARB alone), renal function, sodium and potassium levels, and fluid status (mild diuretic effect) are needed as it is up-titrated to the maximum (97mg/103mg twice a day)/maximum tolerated dose.
Sodium-glucose co-transporter-2 inhibitors (SGLT2i) – dapagliflozin 10mg once a day or empagliflozin 10mg once a day – are also approved by NICE for managing HFrEF. Pharmacists may be more familiar with these medicines for glucose management in type 2 diabetes. However, trial data now support their use in HFrEF patients with or without type 2 diabetes. In the trials, as well as reducing mortality, there was an early and significant reduction in symptoms and hospital admissions.
Patients should be counselled on the common side-effects such as an increased risk of urinary frequency and/or urinary tract infections, genital thrush, and the small risk of serious soft tissue infection of the genital area (Fournier’s gangrene). In addition patients, particularly those with diabetes, should be advised regarding sick day rules (stopping treatment if unwell and until eating/drinking normally) and the signs of ketoacidosis, which may occur even if blood glucose is not raised.
Potassium binders – sodium zirconium cyclosilicate (Lokelma) or patiromer (Veltassa) may enable continued use and up-titration of the prognostic medicines already prescribed (ACEI/ARB/ARNI and MRA) where dosing is limited by hyperkalaemia. These will be specialist initiated with transfer of care to primary care once the patient is stabilised.
Other options
If, despite the above measures, patients are still symptomatic, specialist advice is needed and treatment options might include:
- Consideration for a pacing device that may help improve cardiac contractility +/- a defibrillator function capable of delivering an electric shock if the patient experiences a ventricular arrhythmia. A specialist will advise if the patient fulfils the criteria for such a device
- Addition of ivabradine to improve heart rate control. Ivabradine slows conduction via the I-f channel resulting in a reduction in heart rate. This may be prescribed in addition to the maximum tolerated dose of a beta-blocker for patients in sinus rhythm whose heart rate is still above 75 beats/minute
- Use of hydralazine and nitrates or digoxin for selected patients
- Administration of intravenous iron for patients with iron deficiency, which may improve symptoms and quality of life.