In Clinical
Follow this topic
Bookmark
Record learning outcomes
In November, the British Thoracic Society, NICE and the Scottish Intercollegiate Guidelines Network is due to issue new guidelines covering asthma diagnosis, monitoring and management.
As I know all too well, a serious asthma attack is terrifying and a sobering reminder of one’s mortality. Thousands share my experience.
Asthma + Lung UK notes that asthma deaths in the UK increased by 23.7 per cent between 2014 and 2022. Four people now die from asthma each day and attacks led to 54,869 hospital admissions in the UK during 2021/22. Yet the charity also found that 31 per cent of patients are disengaged from their asthma management, increasing their risk of a potentially life-threatening attack.
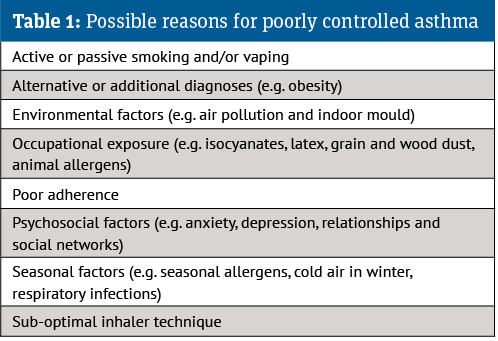
While they exclude the management of severe asthma and acute attacks, the new guidelines aim to improve diagnosis accuracy, help people control their asthma and reduce the risk of asthma attacks. Before considering introducing or adjusting medication, they suggest addressing reasons for uncontrolled asthma (see Table 1).
Complete control is defined as “no daytime symptoms, no night-time awakening due to asthma, no asthma attacks, no need for rescue medication, no limitations on activity including exercise, normal lung function and minimal side-effects from treatment”.
Therapeutic advice
The draft guidance emphasises that healthcare professionals (HCPs) should not prescribe short-acting beta-2 agonists (SABA) to asthma patients of any age without concomitant inhaled corticosteroids (ICS). After starting or adjusting a patient’s medicines, their response should be reviewed after eight to 12 weeks.
Generally, the guidelines represent a move away from separate SABA and ICS inhalers. They suggest a low-dose ICS/formoterol (a long-acting beta-2 agonist) combination inhaler as needed to relieve symptoms in patients aged 12 years and over with newly diagnosed asthma. Not all ICS/formoterol inhalers are licensed as relievers for mild asthma, so use of some would be off-label.
Patients aged 12 years and over whose asthma is inadequately controlled on low-dose ICS/ formoterol combination inhalers can be offered as needed MART (low-dose maintenance and reliever therapy). HCPs can also suggest low-dose MART for patients who present with highly symptomatic asthma (e.g. regular nocturnal waking) or after a severe exacerbation. If asthma is not adequately controlled on low-dose MART, moderate-dose MART should be offered.
If this does not control the asthma, the addition of a leukotriene receptor antagonist (LTRA) could be considered for at least three months (unless side-effects develop) and stop if ineffective. HCPs should consider adding a long-acting muscarinic receptor antagonist (LAMA) to moderate-dose MART alone or plus an LTRA if an LTRA proves ineffective. Again, patients should try the LAMA for at least three months, unless side-effects emerge, and stop if ineffective. Patients should be referred to a specialist if their asthma is not controlled despite treating with moderate-dose ICS, LABA, LTRA and a LAMA.
It is important to ask about side-effects and report these using the Yellow Card system. My asthma tends to be worse in winter, underscoring the importance of asking about seasonality.
A couple of winters ago, my control was far from optimal. My GP persuaded me to try ICS/LABA. I developed severe gastro-oesophageal reflux that woke me more often than the asthma. OTC antacids didn’t help. However, the reflux resolved once I reverted to ICS and SABA in separate inhalers. I can’t prove causality, but my reflux reinforces that HCPs should proactively ask about adverse events and be prepared to change treatments and help address any issues.
Paediatric care
The draft guidelines suggest offering twice daily paediatric low-dose ICS with a SABA as needed as initial treatment for children aged five to 11 years with newly diagnosed asthma. If this does not offer optimal control, paediatric low-dose MART could be considered, provided the patient is able to manage MART. At the time of writing, no asthma inhalers were licensed for MART in children younger than 12 years, so this use would be off-label.
Patients aged five to 11 years should be referred to a specialist if their asthma is not well controlled on paediatric moderate-dose MART or paediatric moderate-dose ICS/LABA maintenance treatment. The draft summarises the intervening steps, management of children younger than five years and other groups (e.g. pregnant women), as well as transitioning from pathways advocated in previous versions of the guidelines.
Inhaler choice
HCPs should suggest an asthma inhaler only after assessing the patient’s technique and considering their preference. Inhaler technique and adherence should be checked at each review. All other things being equal, HCPs should suggest the inhaler with the lowest environmental impact and encourage patients to bring used inhalers back for disposal.
Caution is needed with generic substitutions that alter inhaler size and patient technique checked if there is a change. I was unable to co-ordinate actuation and inhalation when switched to a smaller, generic SABA. The GP practice pharmacist now prescribes my SABA by brand.
Inhalers are increasingly high-tech. The draft guidance does not recommend routinely using digital inhalers, although these may be helpful in cases of poor adherence to maintenance therapy or if referral for biologics is being considered.
Stepping down
At the annual review, HCPs should discuss the potential risks and benefits of decreasing maintenance therapy with the patient if their asthma is well-controlled.
The guidance suggests stopping or reducing the dose in a sequence that reflects effectiveness when introduced, side-effects and patient preference. For example, those using low-dose ICS alone or low-dose MART could step down to low-dose ICS/formoterol as needed. HCP and patient should agree how to monitor and review therapy.
The draft guidelines are available at nice.org.uk/guidance/indevelopment/gid-ng10186