Patients urged to return adrenaline pens to pharmacies
In News
Follow this topic
Bookmark
Record learning outcomes
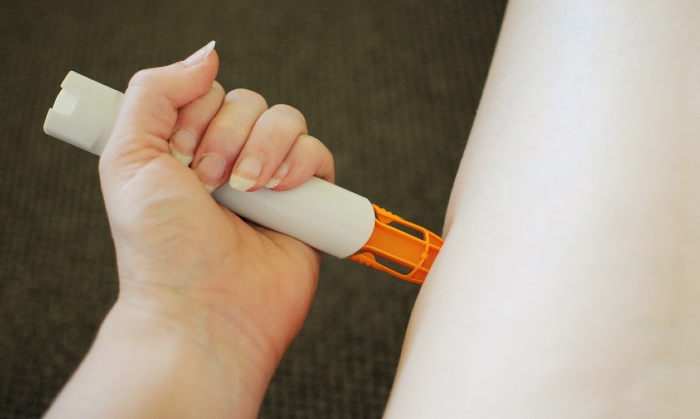
The Medicines and Healthcare products Regulatory Agency (MHRA) has told patients to return Emerade 150-microgram adrenaline pens to their pharmacy over concerns that some of the devices are faulty.
Pharmaswiss, an affiliate of Bausch & Lomb UK Limited, recalled all unexpired batches of the pens after an error was discovered in a component of the auto-injector that could prevent them from delivering adrenaline.
The recall also applies to pens that are held by schools.
The MHRA urged healthcare professionals in primary, secondary and specialist healthcare who prescribe or supply adrenaline auto-injectors to review patients to ensure their device is appropriate “in line with existing guidance.”
The regulator also said healthcare professionals should inform patients and carers to check the expiry date and order a new prescription to replace their Emerade 150-microgram adrenaline pen “in an alternative brand.”
Pharmacists, along with doctors and nurses, have been instructed to train patients and carers to use the new pens.
Patients, the MHRA added, should return their adrenaline pens to the pharmacy “only when they have two alternative adrenaline auto-injectors in their possession.”
The MHRA said: “Pharmacists and pharmacies who receive Emerade 150-microgram auto-injectors from patients should quarantine the pens and return to them to their supplier using the supplier’s approved process.”
